Mythocondro®
Mythocondro® is a fermentation-derived form of chondroitin sulfate, an ingredient linked with superior joint health. Chondroitin sulfate has become widely used as a dietary supplement to help keep joints healthy and promote healthy cartilage.
Chondroitin sulfate has been traditionally sourced from animals, but concerns over cross-contamination and manufacturing processes, as driven demand for a non-animal source of this key nutrient. Gnosis by Lesaffre is proud to fulfill this demand with its revolutionary ingredient Mythocondro®.
- Product category
Active ingredients
- Market segments
-
Digestion & Gut
Mobility & Joint
- Health benefits
-
 Digestive Comfort
Digestive Comfort Joint
Joint Sports Nutrition
Sports Nutrition
- Allergens and
certificates Gluten-free
Lactose-free
Non GMO
 Vegetarian and Vegan
Vegetarian and Vegan
- Download
- Download our product list
Mythocondro® is totally safe, pure, and highly bioavailable
We can guarantee the high quality and purity profile of Mythocondro® because of our controlled, reliable, and replicable fermentation processes. No prions nor animal viruses can be present thanks to its fermentation-derived origins, there is no possibility prions or animal-based viruses in this ingredient. It is free from allergens and GMOs.
Keeping our joints happy and healthy

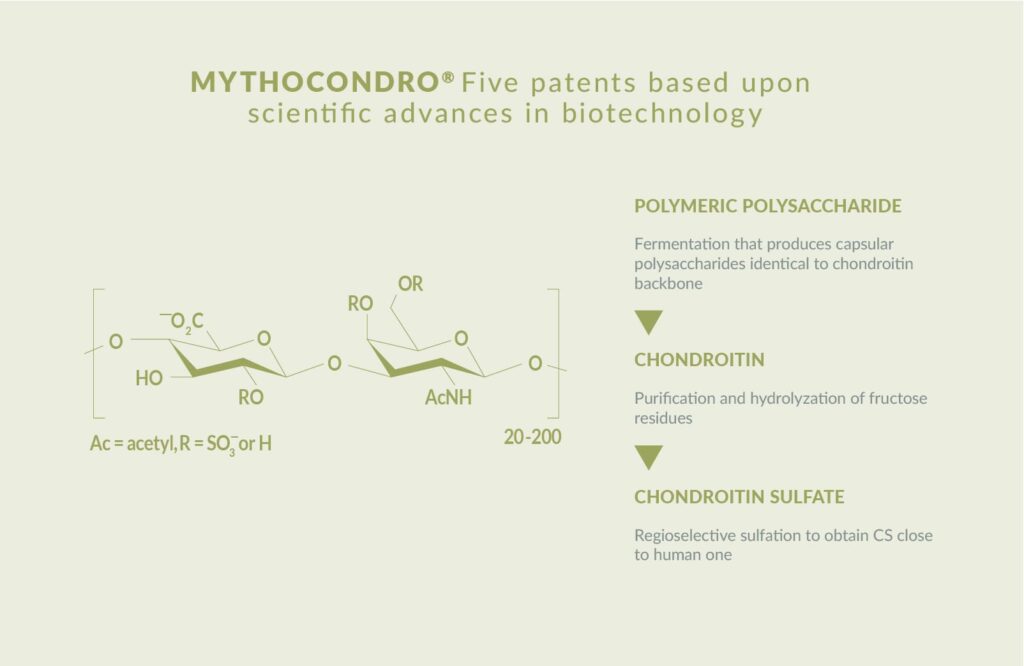
A revolutionary fermentation-derived chondroitin sulfate
Mythocondro has five patents based on scientific advances in bioavailability. The ingredient is also compliant with the USP monograph of chondroitin sulfate sodium.
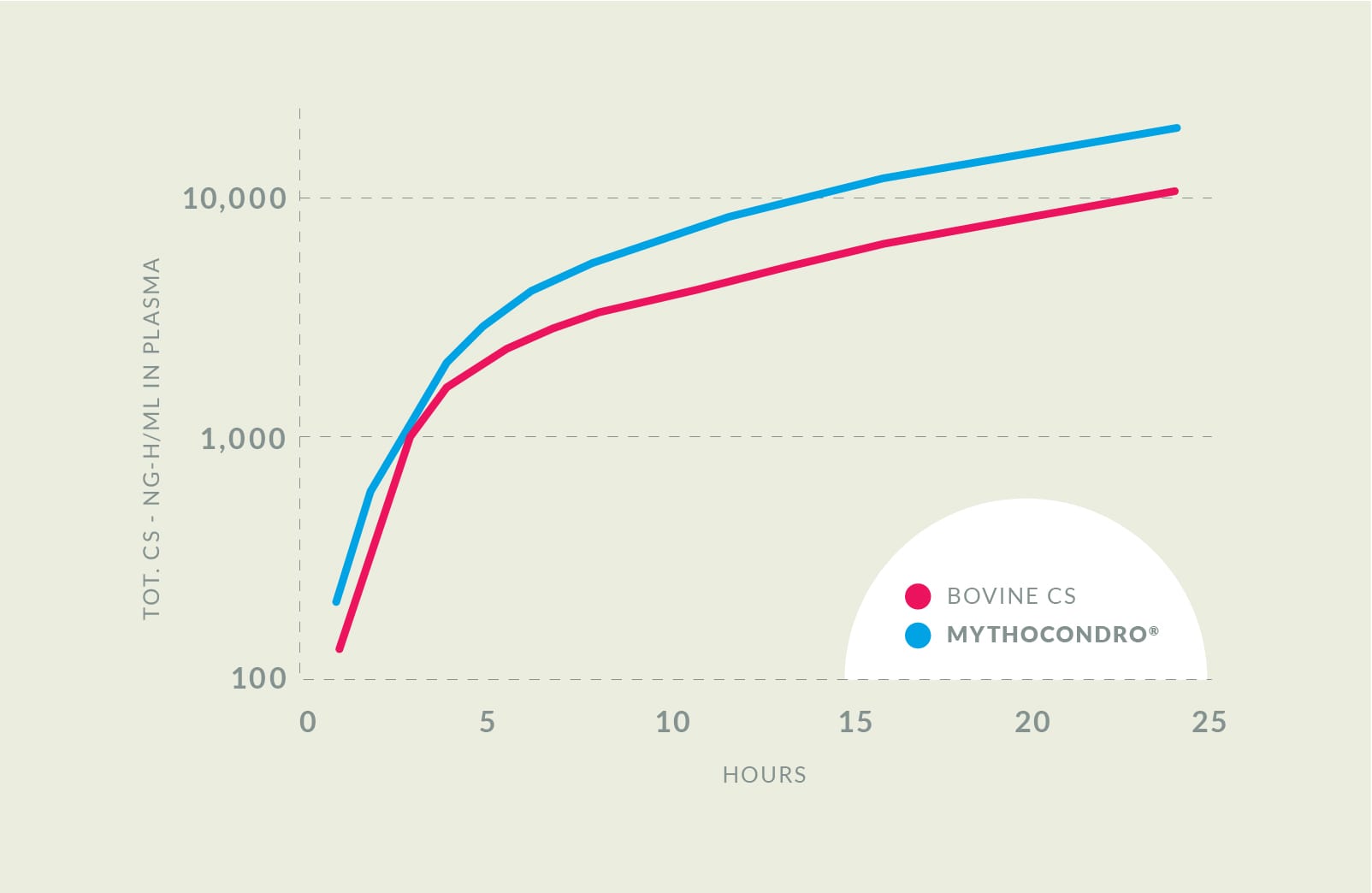
Enhancing bioavailability by 43%
In a clinical trial, Mythocondro showed a higher bioavailability over cattle-derived chondroitin sulfate, meaning that dosages can be lower for the same effect. Even five hours after administration, the plasmatic concentration of Mythocondro remained 89% higher than the animal-derived option.
Related products
Tt enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Titre thématique
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Et, egestas tempus tellus etiam sed. Quam a scelerisque amet ullamcorper eu enim et fermentum, augue. Aliquet amet volutpat quisque ut interdum tincidunt duis.
Frequently asked questions
Can’t find what you are looking for?
Latest news & events

- Adonat
- Digestion & Gut Health
- Leaflet
- Mar 25, 2024
Adonat® Premium SAMe Showcased at Nutraceuticals Europe Summit & Expo 2024: «Investing in Well-being and Longevity»
Gnosis by Lesaffre participated in Spain’s foremost trade event, the Nutraceuticals Europe Summit &...

- MenaQ7®
- Mobility & Joint Health
- News
- Feb 23, 2024
NEW MenaQ7® Matrix Brochure: Clean, Stable & Protected K2
Get the latest insight on how to effortlessly formulate complex ingredient combinations, while ensuring...

- Adonat
- Digestion & Gut Health
- Mobility & Joint Health
- Feb 08, 2024
Adonat® Premium SAMe Sets Apart in The Market, in The Comparative Quality-Based Assessment of SAMe Ingredients
A comparative quality-driven analysis carried out on different SAMe ingredients in the market has...




